What are teletrials?
Teletrials, also known as digi trials, are when a clinician at a primary site uses telehealth (videoconference or telephone calls) to enrol, consent, treat and/or follow up patients at another site, referred to as a satellite site. However, much of the responsibility for the clinical trial remains at the primary site, with the primary site clinician overseeing the conduct of the clinical trial at the satellite site. This substantially reduces the amount of work and oversight required by a clinical trial sponsor, meaning that satellite sites may be activated for a clinical trial at a lesser expense than full activation of the primary site.
The exact division of responsibilities is different for each situation, and subject to contracted delegated responsibilities and duties between the satellite and primary site. The primary and satellite site(s) are referred to as a teletrials cluster.
Satellite sites are often assumed to be sites in remote or regional locations that lack the capacity, infrastructure and expertise to conduct clinical trials on their own, but a satellite site could also be another metro site, or a regional site that has an established clinical trial infrastructure and portfolio. A cluster could potentially also comprise of one main hospital in a state that is activated as a primary site, and several other major hospitals acting as satellite sites.
Why teletrials?
Clinical trials have many benefits. They contribute to medical evidence to improve standards of care, and they often expand treatment options or provide care with less variation. It is known that even patients who are on a placebo arm of a clinical trial, have better outcomes than those patients not enrolled in a clinical trial.
However, clinical trial are expensive to conduct, and hospitals will be selected for participation based on their capacity and expertise to undertake the trial and ability to recruit patients. This can mean that larger treatment centres with many specialist clinicians attract many clinical trials, and patients that are treated at smaller centres may not benefit from this option. In practice, this often means that metro hospitals will conduct clinical trials, and if rural or regionally located patients wish to participate, they may need to travel or stay closer to the metro hospital so they can attend study visits that are part of the clinical trial protocol. The extra burden of travel can mean that some patients decline clinical trial participation.
As research advances, eligible populations for clinical trials in many diseases are becoming smaller, meaning that opening a clinical trial at several large treatment centres can no longer guarantee timely recruitment. Clinical trials that don’t recruit in a timely fashion can lose relevance or equipoise as other research and treatment advances outside the trial, are costly to run and may need to close early. Clinical trials that close early for poor recruitment generally don’t reach a statistically significant result, and thus fail to contribute meaningfully to treatment advances.
For other rare diseases, it simply may not be feasible to open the trial at enough hospitals to reach the required recruitment numbers to answer the question.
For a country like Australia, with vast geographical distances and comparatively low population density, teletrials are seen as a solution to ensure that Australia continues to attract major international clinical trials, and can recruit sufficient numbers to continue to conduct locally conceived and designed clinical trials, such as the investigator-initiated clinical trials conducted by TOGA.
Where is Australia with the implementation of teletrials?
In 2017, COSA together with industry, government and collaborative clinical trial group partners, conducted a pilot teletrial in Queensland. The pilot was a success and Australia has been mobilising teletrials ever since.
The government has released a set of principles and national SOPs, modelled on the pilot teletrial completed by COSA. VCCC have also released SOPs and resources designed from this pilot.
Medicines Australia have released many teletrials resources including a teletrials-specific template contract that can be used between the primary and satellite site, and a suggested payment schedule. Medicines Australia have also released a specific teletrial patient informed consent form.
![]() The Australian government has invested $125 million in a Rural, Regional and Remote Clinical Trial Enabling Infrastructure Program, funded under the MRFF, that will help improve access to equipment, services and clinical trials for people living in the regions.
The Australian government has invested $125 million in a Rural, Regional and Remote Clinical Trial Enabling Infrastructure Program, funded under the MRFF, that will help improve access to equipment, services and clinical trials for people living in the regions.
$75.2 million of this has been provided to Queensland Health, led by Professor Sabe Sabesan in Qld, to coordinate teletrials establishment in Qld, WA, SA, NT, VIC and TAS.
$30.6 million has been provided to the Office of Health and Medical Research in NSW to establish teletrials in NSW and ACT as part of their Rural, Regional and Remote Clinical Trial Enabling Program.
$18.6 million has been provided to Border Medical Oncology Unit, led by A/Prof Craig Underhill in Vic, to coordinate teletrials establishment in regional VIC.
Key to this MRFF funding is the establishment of Regional Clinical Trial Coordinating Centre (RCCCs) in VIC-TAS, SA-NT, WA, Qld, NSW-ACT, comprised of key stakeholders in the clinical trials sector, ideally including clinicians, industry representatives and those from the investigator-initiated clinical trial sector. The role of the RCCCs is to facilitate the establishment of teletrials within each jurisdication’s governance system, and also ensuring harmonisation to facilitate the conduct of national multicentre clinical trials. These RCCCs will be advised by experts in clinical trial operations from industry, and CCTGs, and by clinicians and consumer representatives.
Outside this MRFF funding, the TrialHub, Vic, has provided $25 million in funding in 2019 to establish teletrials between Alfred and six Victorian hospitals; Latrobe Regional Health, Bendigo Health, Mildura Base Public Hospital, Peninsula Health (Rosebud), Bass Coast Health, and Northern Health.
What is TOGA’s role in the mobilisation of teletrials?
TOGA has activated teletrials in ASPiRATION, the ASPiRATION substudies and ALKTERNATE, but has also received ILC2 grant funding to mobilise new clusters for lung cancer clinical trials, to actively implement review of developing clinical trial concepts for amenability to teletrials, as this is a stage of development where design could potentially be modified to better accommodate teletrials, and to raise awareness of teletrials. To assist with clinical trial design that is more amenable to teletrials, TOGA has developed a checklist that is completed for all developing TOGA trials. If possible, the design may be amended to better accommodate teletrials.
The mobilisation of teletrials clusters centres around bringing together lung cancer clinicians/TOGA members of potential clusters, and briefing them of national progress, facilitators and any potential barriers, focusing particularly on facilitating progress through governance requirements. Ideally these establishing clusters would then lobby their particular health service for implementation of the cluster to a point where they can respond to site selection surveys as a teletrials cluster.
TOGA is also coordinating with the other Cancer Cooperative Trials Groups to stay abreast of establishing clusters, liaising with the NHMRC-CTC to adapt existing procedures to accommodate teletrials and seeking progress updates from key leaders of the MRFF-funded activities.
TOGA’s mapping of teletrial clusters
In the first half of 2023 TOGA surveyed its membership in order to establish a preliminary map of existing teletrial clusters as well as provide an overview of potential clusters and those in the process of being established. While the data that we obtained is limited by the participants’ insight and personal views we were able to gain a valuable understanding of the evolving landscape of teletrials in Australia.
Depicted in Figure 1 is the mapping of the respondents’ answers on current teleclusters within Australia. While it was expected that the majority of teletrials are linked to major hospital hubs, it came to our suprise that these were only identified within three states.
In comparison, our survey results on proposed and developing teleclusters depicted in Figure 2 show promising preliminary findings that this map is shifting to include other states within Australia, as well as uncovering development of clusters that cross state borders.
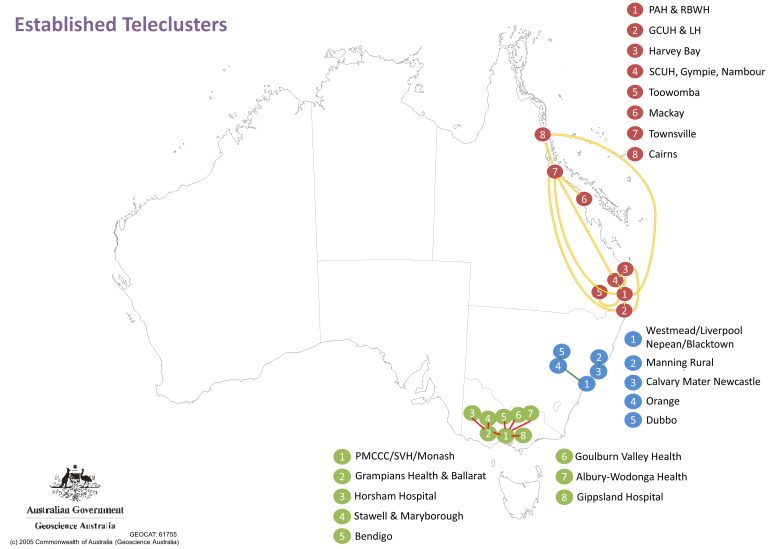
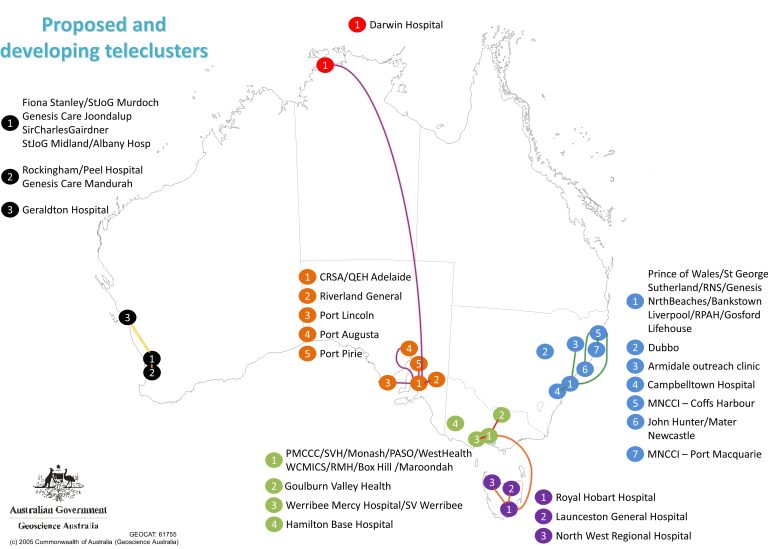
Conclusions based on TOGA’s findings
There are several limitations to keep in mind when interpreting these findings:
- The data is based on a small sample size of 33 responses and is limited to the respondents’ knowledge of telecluster status.
- Clusters limited to hospitals within major cities are not detailed.
However, regardless of the limitations TOGA believes that our research has not only identified the expanding and evolving networks of teleclusters but identified potential sites (such as Hamilton Base hospital in Figure 2) that are not part of teleclusters but could be recruited to expand the network.
If you want to reach out to us in order to help us maintain or update these maps, you can contact us on info@thoraciconcology.org.au
Additional Resources
- COSA teletrials project
- VCCC teletrials resources
- Collins I, Burbury K, Underhill C. Teletrials: implementation of a new paradigm for clinical trials. 2020 MJA 213:263 https://doi.org/10.5694/mja2.50741
- Implementing teletrials with A/Prof Ian Collins MJA Podcast 2020 Episode 35.
- The future of cancer clinical trials in regional Victoria. VCCC Monday Lunch Livestream
- Teletrials closing the geographic gap in cancer mortality. Insight Issue 34; 31 August 2020
- TOGA checklist for teletrials